Sowing dry seeds
If you want to flask dry seeds, you have to wait till your capsule opens. When the capsule starts to dehisce, cut the capsule and shake the dry seeds out on a sheet of paper. Before you start sterilizing your seeds you should remove all contaminations (parts of the capsule, pollen tubes, ...)
necessary tools
- laminar flow hood
- alcohol burner
- forceps
- test tubes
- glass funnel
- pipette
- sealing film or rubber plug
- scalpel
- glass petri dish
necessary articles of consumption
- flasks (e.g. test tubes) containing media
- kitchen paper
- cotton pads
- 70% ethanol
- bleach solution (e.g. DanKlorix, Clorox, ...)
- sterile filter paper
- sterile distilled water
- dishwashing detergent
- cotton pads
Advantages of using dry seeds
Disadvantages of using dry seeds
|
Preparing sterile filter paper and distilled water
Filter paper gets sterile, when you wrap it in aluminium foil and sterilize it together with your flasks in the pressure cooker (see media preparation). The procedure to sterilize distilled water is similar. Put some distilled water into a jar, screw down the cap loosely and cover the cap with aluminium foil. The jar needs to get sterilized in your pressure cooker in the same way as you do it with your flasks.
Preparing the desinfection solution
To reduce the high concentration of your bleach solution to the required 0,14% chlorine, distilled water has to be added. The following formula can be used to calculate the required quantity of high concentrated bleach solution to prepare a given quantity of a 0,14% chlorine solution.
Example:
| Wanted quantity of 0,14% chlorine solution: | 100ml |
| Concentration of the high concentrated solution: | 2,8% |
| Formula: quantityhighconcentrated = (quantitytotal * concentrationlow) : concentrationhigh = (100ml * 0,14%) : 2,8% = 5ml |
In this example you have to put 5ml bleach solution into a beaker and add distilled water till you reach a total quantity of 100ml. This 100ml will have a concetration of 0,14% chlorine.
Add a drop of dishwashing detergent to the desinfection solution to make sure that it gets in contact with the entire surface of the seeds.
Preparing the flasking area
This document describes the procedure of flasking orchid seeds in a LFH (laminar flow hood). Detailed informations how a laminar flow hood works and how to build it are available here. The following photo shows our LFH and all required tools. In the laminar flow hood there should be as less things as possible to reduce the risk of contaminations to a minimum.

The flasking area has to be sterilized before you can start working in it, because contaminations get in contact with the flasking area when the LFH is turned off. We soak a kitchen paper with 70% ethanol and use it to clean the flasking area after turning on the LFH.
Sterilizing the dry seeds
Fill your test tube half-full with your desinfection solution (see above). Add a small quantity of seeds and close the test tube with sealing film or a rubber plug.

In the next 15 minutes agitate the test tube again and again to enable the desinfaction solution to get in contact with each seed.
While the seeds are getting sterilized, clean the glass funnel with a cotton pad, which was soaked with 70% ethanol, and bring it into the flasking area of your LFH (laminar flow hood). Next, put the sterile filter paper into the funnel and use the pipette to put a small quantity of your desinfection solution onto the filter paper - this holds the paper in the filter. Additionally you´ll need the glass petri dish. Clean it with a 70% ethanol soaked cotton pad and place it in your flasking area.

Transferring the sterile seeds onto the media
The following steps must be done in the sterile area (steam). Open test tubes and their cotton plugs have to stay in the steam till the test tube is closed again.
When the seeds spent 15 minutes in the 0,14% chlorine solution, it's time to bring them into their flasks. Open the test tubes with the seeds in your flasking area and pour its content in the glass funnel. Agitate the test tube before pouring its content into the funnel to make sure that the seeds are swimming in the desinfection solution - sometimes they tend to stick at the wall of the test tube.
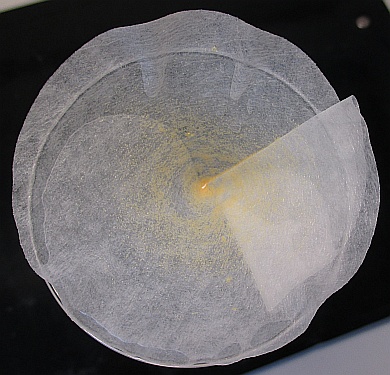
When the chlorine solution is not longer in the funnel, sterile water should be used to rinse the seeds down to the deepest point of the funnel. The sterile water also removes remaining chlorine solution, which can demage the seeds if it stays on the seeds.

Now it's time to flame a forceps and transfer the filter paper from the funnel onto the petri dish.

Open one of your flasks in the flasking area, pick up some seeds with a flamed forceps and bring them into the flask.

Some hints
- open flasks and their lids have to stay in the flasking area till they are closed again
- don´t move around to fast in the flasking area
- don´t speak while you're working in the flasking area
Further care
You should keep your flasks in a bright and warm (20 - 25°) place. It is very important to prevent direct sun, because the seeds in the flasks will become to hot if they get direct sun. Beside nature light, artificial light can be used as well. We prefer T5 fluorescent tubes with light color 865. T5 tubes need less power and they do not get that warm as T8 tubes do.


Author: Thomas Ederer
